
Innovative mobility solutions for a dynamic world
How we enable sustainable mobility
Driven by innovation, Freudenberg is transforming the way people move with our cutting-edge smart mobility solutions. From electric vehicles to intelligent transportation systems, our innovations are designed to make travel safer, more efficient, and environmentally friendly. Discover how we are revolutionizing mobility for a smarter future.
E-Mobility
safe, efficient and durable

Freudenberg is combining material expertise and innovation to make electric mobility safe and durable.
Improvements in performance are another goal. We offer vehicle makers a broad portfolio of solutions, ranging from battery systems to high-performance materials and even efficient pressure management technologies. Freudenberg’s Business Groups are drawing on their decades of experience and their in-depth technical know-how as they actively support the mobility transformation.
Optimized solutions for heavy-duty applications
To meet the growing demands in the heavy-duty sector, Freudenberg has taken its know-how in the battery and fuel cell fields and bundled it into a special Business Group: Freudenberg e-Power Systems.
As a provider of emission-neutral energy systems, Freudenberg e-Power Systems develops innovative solutions for the challenging heavy-duty sector. Our battery and fuel cell systems are specifically designed and customized for buses, trucks, maritime and off-road applications to provide our customers with optimal, safe solutions for every application.
Robust housings, precise thermal management, and smart monitoring systems are delivering reliable performance - even under extreme loads.

XRange Battery Pack
Protection against extreme temperatures
Even beyond heavy-duty applications, Freudenberg is turning to innovative solutions to make battery systems in electric vehicles safer while boosting their performance. Here highly sophisticated materials play a key role.
One example is Quantix® ULTRA. It provides reliable flame protection and limits the spread of potential flames within the battery module. Especially in conductor rails, also known as busbars, the high-performance material decreases the risk of short circuits and helps to regulate temperatures efficiently.
The heat shield provides additional protection. It is a silicone-based elastomer with a waffle-like structure. This protective thermal layer keeps high temperatures from reaching adjacent battery modules.
Special Freudenberg materials also function as thermal barriers in battery compartments. They withstand temperatures of up to 1,200°C and prevent the uncontrollable release of the tiny particles that are produced when a cell is defective.
We have succeeded in combining the worlds of thermoplastics and thermosets.
Dr. Björn Hellbach
Senior Chemical Specialist at Freudenberg Sealing Technologies
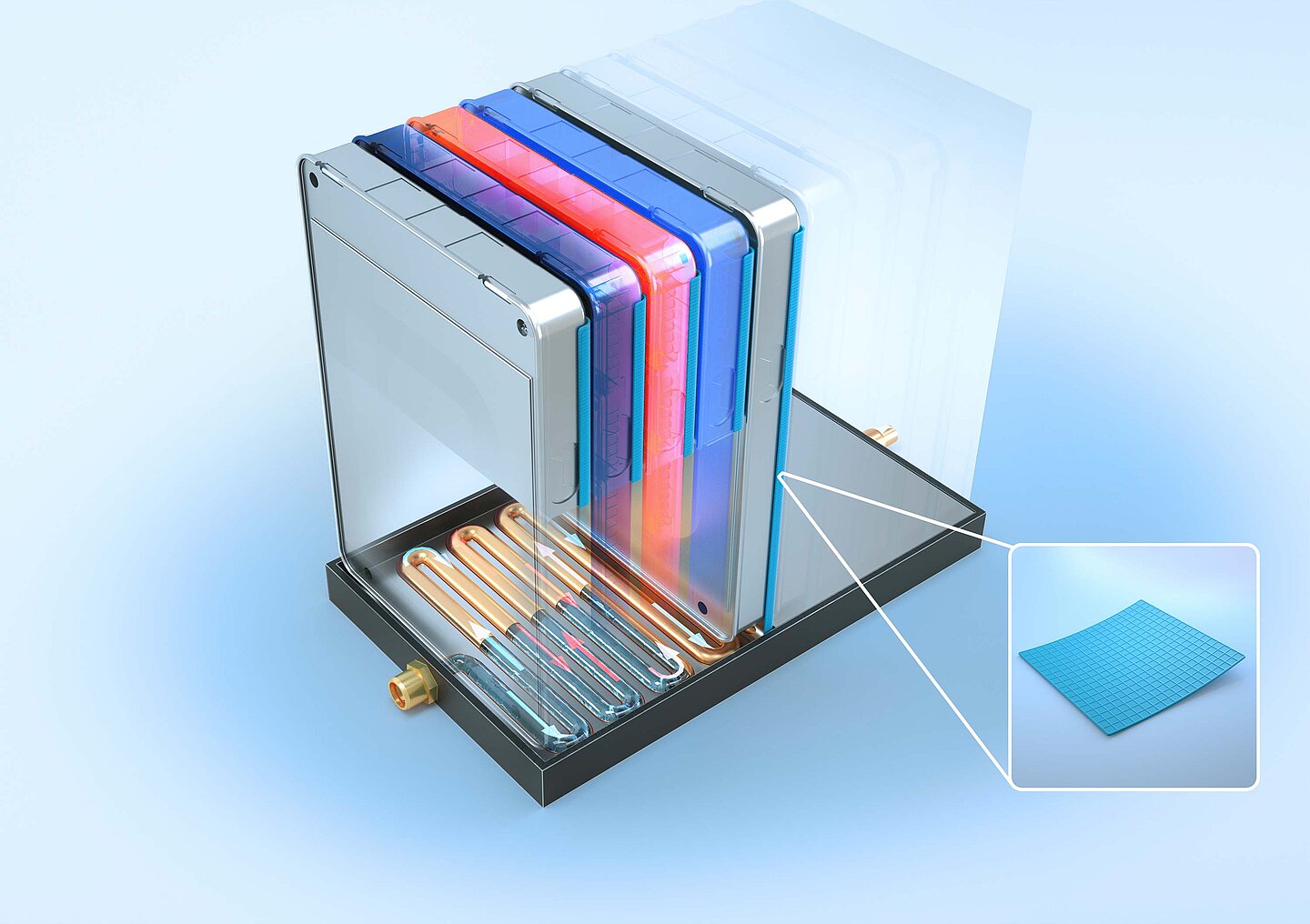
The heat shield provides additional protection. It is a silicone-based elastomer with a waffle-like structure. This protective thermal layer keeps high temperatures from reaching adjacent battery modules.
Efficient pressure management
Aside from thermal protection, thermal management plays a significant role in promoting a long operating life for battery systems. The innovative venting system DIAvent® provides safe pressure equalization within the battery module. During normal operation, a “breathing” membrane enables controlled air exchanges while keeping water and dirt from penetrating. In case of a critical battery event, DIAvent® opens up instantly, draining off gases safely and preventing a dangerous pressure increase.
Protection against exposure to fluids
Protection against exposure to fluids is another important factor affecting the lifespan of a battery system. Humidity or a leaking coolant can damage sensitive electronics and cell modules. Freudenberg's Battery Pack Liquid Absorbers effectively absorb and store any liquid that may appear, preventing potential damage to the system.
Inspiration from nature
High-Speed Evolution
Vibracoustic achieves in just a few days what nature has perfected over millennia. With artificial intelligence, engineering teams develop new vibration dampers more precisely, faster, and more sustainably.
AI-driven development for maximum efficiency
At Vibracoustic, engineering teams continuously improve their products, extending the use of AI. The technology analyzes hundreds of parameters simultaneously and creates up to 200 possible rubber designs within 24 hours. Only the most promising concepts are pursued further, saving time while increasing precision and efficiency.
Speed as a competitive advantage
“Vibracoustic is a global leading manufacturer of NVH (noise, vibration, and harshness) solutions for the automotive industry. We want to accelerate our development times to make sure that we maintain our leading position.” explains Olivier Leclercq, Director Innovation & Digitalization at Vibracoustic. Through AI-supported processes, Vibracoustic wants to respond flexibly to new, powertrain concepts and market demands while setting new benchmarks in NVH development.
Tailored solutions for every vehicle class
Whether it's an electric SUV, a city car, or a hybrid transporter, AI identifies the optimal rubber shape for each application. Practical tests confirm the high degree of consistency between predictions and reality, proving the reliability of the technology and the interaction between humans and machines.
Data-driven into the future
Design automation and AI go hand in hand at Vibracoustic. Automated workflows reduce manual effort, collect data based on the FAIR* principles, and create a foundation for data-driven engineering. With global use, easy accessibility, and continuous extension of design automation, the engineering teams save valuable time and are optimally positioned for the future.
What are the FAIR principles?
The FAIR principles ensure that data is findable, accessible, interoperable, and reusable.
A look ahead
“We are already steadily improving our model with the integration of experimental tests and material data. Our goal is to apply the technology to all the important acoustic rubber metal products.” said Nicolas Le Maoût, FEA (Finite Element Analysis) Engineer at Vibracoustic.
While nature takes millions of years to evolve, Vibracoustic achieves evolution in fast forward – showing how AI is shaping the mobility of tomorrow.

















